Calorimetry: How many grams of ice at - 13°C must be added to 711 grams of water that is initially at a temperature of 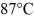 to produce water at a final temperature of
to produce water at a final temperature of 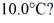 Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg · °C and of ice is 2050 J/kg · °C. For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg · °C and of ice is 2050 J/kg · °C. For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Definitions:
Management-By-Objectives
A strategic management model focusing on setting and achieving specific objectives within an organization.
Efficiency
The ability to accomplish a job with a minimum expenditure of time and effort.
Resource Inputs
The materials, capital, energy, information, and human skills required to produce goods or services.
Measures For Efficiency
Metrics or standards used to evaluate how effectively and economically tasks or processes are performed.
Q3: Resistors in combination: Three resistors having resistances
Q6: What are the other names for a
Q10: Ohm's law: A cylindrical wire of radius
Q15: Physical pendulum: A large stick is pivoted
Q27: Electric field and potential : Suppose a
Q32: Gravitational potential energy: Two planets having equal
Q45: Resistivity: What length of a certain metal
Q50: Components: A teacher sends her students on
Q52: Doppler effect: You are driving along a
Q101: Ideal gas law: A hot air balloon