Mean free path: Assuming the radius of diatomic molecules is approximately 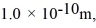 for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K.
for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K.
Definitions:
Collective Agreement
A written contract negotiated between an employer and a union representing the employees, outlining terms and conditions of employment.
Technical Requirements
Specifications or criteria that must be met by a system, design, product, or service to fulfill its intended purpose.
Cultures
The shared values, beliefs, norms, and practices that define a group, organization, or society, shaping its members' behaviors and interactions.
Leadership Style
Refers to a leader's method of providing direction, implementing plans, and motivating people.
Q5: First law of thermodynamics: In an isochoric
Q6: First law of thermodynamics: The pV diagram
Q6: Simple harmonic motion: A machine part is
Q10: Motion of a charged particle: An electron
Q15: Components: A helicopter is flying horizontally with
Q28: Phase changes: A 406.0 kg copper bar
Q33: Potential energy of point-charges: Suppose you have
Q33: Flow rate: Water is flowing in a
Q41: Resistivity: Nichrome wire, often used for heating
Q93: Beats: Two motors in a factory are