Quantity of heat: A 648-g empty iron kettle is put on a stove. How much heat. in joules. must it absorb to raise its temperature from 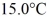 to
to 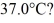 (The specific heat for iron is 113 cal/kg ∙ °C, 1 cal = 4.190 J)
(The specific heat for iron is 113 cal/kg ∙ °C, 1 cal = 4.190 J)
Definitions:
Total Expenses
The sum of all costs and expenses incurred by a business or organization during a specific period.
Accrued Wages
Wages that have been earned by employees but have not yet been paid or recorded in the financial statements.
Pay Period
The interval of time for which an employee’s wages are calculated, such as weekly, bi-weekly, monthly, etc.
Depreciation
The systematic reduction in the recorded cost of a fixed asset to allocate its expense over its useful life.
Q3: Carbon and silicon are examples of _.<br>A)
Q5: Gravitational force: An astronaut is in equilibrium
Q27: Ampere's law: A tube with a 3.0-mm
Q34: Electric field in a wire: A narrow
Q35: Resistivity and temperature: The temperature coefficient of
Q37: Gravitational force: Three identical very small 50-kg
Q51: Buoyancy: A person who weighs 550 N
Q53: Field due to a long wire: Two
Q77: Intensity: The intensity of sunlight falling on
Q98: Standing sound waves: A pipe that is