Calorimetry: How many grams of ice at - 13°C must be added to 711 grams of water that is initially at a temperature of 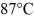 to produce water at a final temperature of
to produce water at a final temperature of 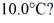 Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg · °C and of ice is 2050 J/kg · °C. For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg · °C and of ice is 2050 J/kg · °C. For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Definitions:
Assessing
The process of evaluating or estimating the nature, ability, or quality of something or someone.
Diagnosing
The process of identifying a disease or condition from its symptoms and clinical signs.
Research Domain Criteria
A framework for research into mental disorders that integrates genetics, neuroscience, and behavior to better understand the complexities of mental health.
National Institute of Mental Health
The lead federal agency for research on mental disorders, aiming to transform the understanding and treatment of mental illnesses.
Q2: If two insulated wires were to melt
Q2: What are the ampere, volt, and ohm?
Q7: Thermal expansion: Two identical concrete slabs lie
Q21: Potential due to point-charges: Two +6.0-μC point
Q25: Force on parallel wires: As shown in
Q36: Buoyancy: If you double the pressure on
Q39: Coulomb's law: In the figure, all the
Q50: Carnot engine: A perfect Carnot engine operates
Q98: Conduction of heat: A heat conducting rod,
Q107: Sound level: An enclosed chamber with sound