Entropy: What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol∙K) . 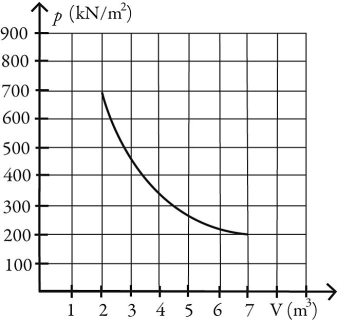
Definitions:
H1-B Provision
A provision of the U.S. immigration law that allows the annual entry of 65,000 high-skilled workers in “specialty occupations” such as science, R&D, and computer programming to work legally and continuously in the United States for six years.
High-Skilled Workers
High-skilled workers are individuals who have advanced qualifications, training, or expertise, making them capable of performing complex tasks.
Specialty Occupations
Jobs or professions that require the application of specialized knowledge, often necessitating a higher degree of education or experience.
Population Growth
The increase in the number of individuals in a population, often measured by the percentage increase per year.
Q7: Potential energy of point-charges: Suppose you have
Q8: Two technicians are discussing torque wrenches. Technician
Q19: Current: The figure shows a 2.0-cm diameter
Q19: Molecular speeds: What is the average translational
Q21: Simple pendulum: A frictionless pendulum released from
Q26: Energy in capacitors: Two square air-filled parallel
Q27: First law of thermodynamics: When a fixed
Q41: Components: Which of the following is an
Q61: Conduction of heat: Two metal rods, one
Q82: Doppler effect: A carousel that is 5.00