Entropy: A 810-g quantity of ethanol, in the liquid state at its melting point of 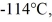 is frozen at atmospheric pressure. The heat of fusion of ethanol is 1.04 × 105 J/kg, the molecular mass is 46.1 g/mol, and the ideal gas constant is R = 8.314 J/(mol∙K) . The change in the entropy of the ethanol as it freezes is closest to
is frozen at atmospheric pressure. The heat of fusion of ethanol is 1.04 × 105 J/kg, the molecular mass is 46.1 g/mol, and the ideal gas constant is R = 8.314 J/(mol∙K) . The change in the entropy of the ethanol as it freezes is closest to
Definitions:
Measurement Strategy
A plan or approach designed to quantify processes, outputs, or outcomes, enabling assessment and improvement.
Integrated
Describes systems or methods that are combined in order to function as a coherent whole, often referring to marketing strategies that use multiple channels cohesively.
Campaign
An organized course of action to achieve a particular goal, often used in marketing or political contexts.
Measurement And Evaluation
The process of determining the effectiveness or value of a process, system, or activity, usually involving the collection and analysis of data to assess outcomes and make improvements.
Q8: Cycles per second are expressed in _.<br>A)
Q9: Oscilloscopes use what type of lead connector?<br>A)
Q14: Capacitors in combination: When two or more
Q15: Energy in capacitors: An ideal air-filled parallel-plate
Q17: Interference: Two in-phase loudspeakers that emit sound
Q25: Weight and g: From what height above
Q28: Unit vectors: Vector <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8274/.jpg" alt="Unit vectors:
Q35: Charged ring: A charge Q = -610
Q35: Scalar (dot) product: The value of the
Q37: Shock waves: Shock waves occur when<br>A) the