Entropy: What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol∙K) . 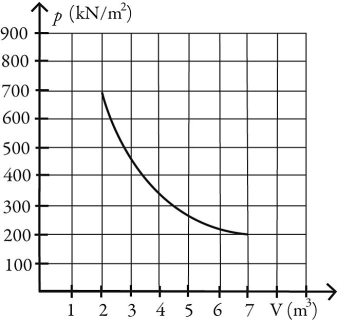
Definitions:
Spatial Intelligence
The ability to visualize, manipulate, and reason with objects in three-dimensional space.
School Setting
A school setting is the environment in which formal education takes place, including the physical classrooms, the teaching methods, social interactions, and the culture of the institution.
Intelligence Tests
Standardized tests designed to measure a person's ability to think, reason, and solve problems.
Triarchic Theory
A theory of intelligence proposed by Robert Sternberg that suggests there are three types of intelligence: analytical, creative, and practical.
Q2: An oscilloscope display is called a _.<br>A)
Q2: What is the difference between a wire
Q2: How can a diode be used to
Q12: Refrigerators: A refrigerator removes heat from the
Q17: Simple harmonic motion: A restoring force of
Q33: Coulomb's law: Three point charges are placed
Q36: Molecular speeds: If we double the root-mean-square
Q41: Standing waves on a string: A 2.00-m
Q73: Thermal expansion: The exterior of a supersonic
Q79: Calorimetry: Two experimental runs are performed to