Type of thermodynamic processes: The figure shows a pV diagram for 8.3 g of nitrogen gas (N2) in a sealed container. The temperature T1 of the gas in state 1 is 79°C. What are (a) the pressure p1 of the gas in state 1 and (b) the temperature T2 of the gas in state 2? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of nitrogen is 14 g/mol. 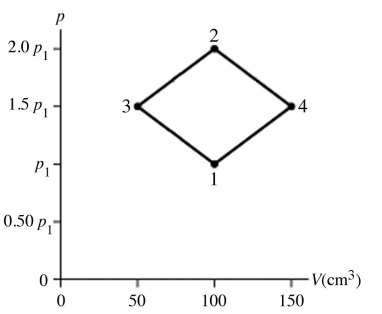
Definitions:
IFRS 9
International Financial Reporting Standard 9, dictating the accounting for financial instruments, including recognition, measurement, and impairment of assets.
Accounts Receivable
Money owed to a business by its clients or customers for goods or services delivered or used but not yet paid for.
Hedge
An investment made to reduce the risk of adverse price movements in an asset, typically involving taking an offsetting position in a related security.
Spot Rate
The current price at which a particular currency can be bought or sold for immediate delivery.
Q1: List three precautions that must be taken
Q2: Entropy: A 2.0-kg block of aluminum at
Q4: Gravitational potential energy: An astronaut is standing
Q9: Operating an alternator in a vehicle with
Q14: Charge on conductors: An irregular conductor carries
Q26: Gauss's law: Which of the following statements
Q40: Parallel plates: The electric field strength in
Q65: Doppler effect: A bat emits a sound
Q67: Sound level: The howler monkey is the
Q118: Mean free path: The mean free path