Type of thermodynamic processes: The figure shows a pV diagram for 0.95 mol of gas that undergoes the process 1 → 2. The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 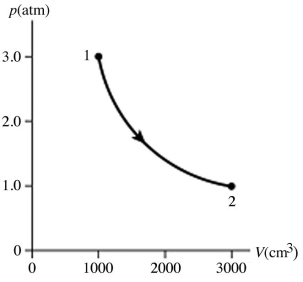
Definitions:
Bank Account
A financial account maintained by a bank or other financial institution in which the financial transactions between a customer and the bank are recorded.
Discounted
A reduction applied to the price of goods, services, or securities, or the process of calculating the present value of a series of future cash flows.
Simple Interest
Interest calculated only on the initial principal of an investment or loan over a specified time period.
Contract
A legally binding agreement between two or more parties outlining obligations, rights, and conditions.
Q4: Why should a battery not be fast
Q7: All of the following are the proper
Q8: Two bulbs are connected in parallel to
Q15: Buoyancy: A circular cylinder of height 1.20
Q40: First law of thermodynamics: A container with
Q48: Coulomb's law: A positive point charge Q
Q50: Mass on a spring: A 2.0 kg
Q53: Components: Vector <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8274/.jpg" alt="Components: Vector
Q97: Thermal expansion: If the temperature of an
Q101: Ideal gas law: A hot air balloon