Molar heat capacities: An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram. The initial pressure is 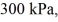 the initial volume is
the initial volume is 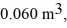 and the initial temperature is
and the initial temperature is 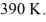 The ideal gas constant is R = 8.314 J/mol ∙ K. The final pressure is
The ideal gas constant is R = 8.314 J/mol ∙ K. The final pressure is 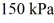 and the final temperature is
and the final temperature is 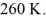 The work done by the gas is closest to
The work done by the gas is closest to
Definitions:
Life Expectancy
The average number of years an individual is expected to live, based on statistical averages for a particular population or demographic.
Health Expectancy
An estimate of how many years a person can expect to live in a state of good health, taking into account morbidity and disability rates.
PPACA
The Patient Protection and Affordable Care Act, a U.S. law enacted in 2010 aimed at expanding health insurance coverage, reducing healthcare costs, and improving healthcare system efficiency.
Personal Mandate
Specific requirements or duties imposed on individuals, often referring to health insurance obligations.
Q1: Technician A says that distilled or clean
Q1: On a wiring diagram, S110 with a
Q3: What tests can be performed to determine
Q11: Calorimetry: A person makes ice tea by
Q27: Simple harmonic motion: Which of following graphs
Q37: Multiple point-charges: Two point charges of +
Q46: Waves on a string: When a weight
Q48: Mass on a spring: A 12.0-N object
Q55: Electric field and potential: If the electric
Q91: Intensity: Two people are talking at a