The following electrolytic cell is a representation of the process used to purify copper metal. How does it work? 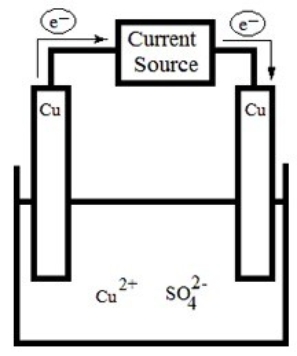
Definitions:
Actual Overhead Costs
The real expenses associated with manufacturing overhead, including all indirect costs incurred.
Overhead Costs Applied
The allocation of overhead expenses, including indirect costs, to specific cost objects like products or services.
Finished Goods
Finished goods are completed products ready for sale, having passed through all stages of production and manufacturing.
Direct Labor Cost
The expense associated with labor directly involved in the production process of goods or services, distinguishing from indirect labor costs like maintenance personnel.
Q1: The air at sea level is ~80%
Q7: Indicate whether the following statement are true
Q14: Indicate whether the following statement are true
Q22: What factors predispose to the development of
Q26: Which of these elements when doped into
Q39: Which type of isomerism is described as
Q51: The reaction of hydrogen chloride gas with
Q94: _ disrupt radio transmission and are responsible
Q102: What is the noxious cloud of smoke
Q115: What is the nuclear binding energy per