Based on the following electrochemical cell, what is the standard reduction potential of metal M at 298 K? (R = 8.314 J/K • mol, F = 96500 C/mol) 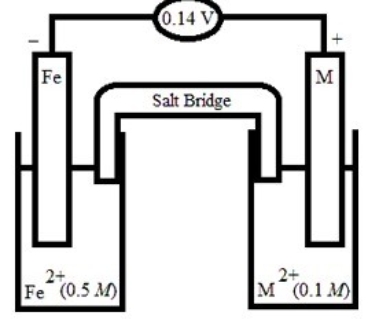
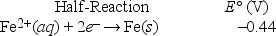
Definitions:
Exponential Growth
A growth rate that becomes ever more rapid in proportion to the growing total number or size.
Innovation
The process of creating new ideas, products, or methods, which improve upon existing standards or introduce novel solutions.
Casual Crowd
A gathering of individuals who come together without a specific purpose or for a short-lived event, often spontaneously.
G-8 Summit
An annual forum for the leaders of eight of the world's major economies to discuss and coordinate economic policy and global issues.
Q16: A solution is prepared by adding 0.10
Q24: A buffer is prepared by adding 1.00
Q24: Which below is a CFC?<br>A) CFCl<sub>3</sub><br>B) CF<sub>2</sub>Cl<sub>2</sub><br>C)
Q41: What is the name given to SO<sub>2</sub>
Q69: Which of these square planar complex ions
Q87: What is the electron configuration of a
Q90: A nuclear reaction's reaction rate is affected
Q97: _ is the layer above the troposphere
Q113: The radioisotope potassium-40 decays to argon-40 by
Q124: For the common allotropes of carbon (graphite