What is E°cell for the following reaction, and is it spontaneous or nonspontaneous under standard-state conditions? 2Fe3+(aq) + 2H2O(l) → H2O2(aq) + 2H+(aq) + 2Fe2+(aq)
Given: 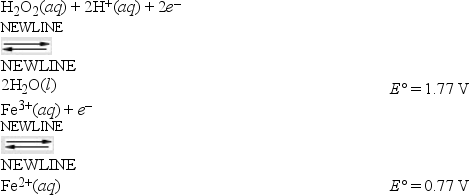
Definitions:
Cultural Diversity
The presence of multiple cultural groups within a society, encompassing differences in race, ethnicity, nationality, religion, and other social factors.
Cross-Culture
Pertaining to or comparing different cultures, or the interaction between individuals from different cultural backgrounds.
Attributions
Explanations for the reasons behind people’s behavior.
Causality
The relationship between a cause and its effect, or understanding why things happen and the consequences that follow.
Q10: Which statement is correct?<br>A) Reaction of ADP
Q31: Nitrogen fixation occurs through atmospheric, industrial, and
Q33: What is the layer of the atmosphere
Q34: If E° for X + e<sup>-</sup> →
Q37: What product forms at the cathode during
Q38: The rate constant of the reaction Cl
Q74: _ is a process used to coat
Q81: The dose unit of ionizing radiation is
Q85: As a chemical reaction proceeds toward equilibrium,
Q106: Consider the following equilibria: 2SO<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg"