What is the minimum voltage required for the electrolysis of 1.0 M NaCl in neutral solution? 2H2O + 2Cl- (1.0 M) → H2(1 atm) + Cl2(1 atm) + 2OH-(1 × 10-7 M) 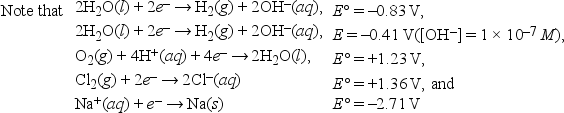
Definitions:
Judgmental Factors
Elements that require personal assessment and decision-making in the valuation of assets, determination of liabilities, or other financial reporting activities.
Static Budget
A budget that remains unchanged over the period it covers, regardless of changes in sales volume, production levels, or other external factors, often used for fixed expenses.
Control Costs
The practice of managing and regulating expenses to ensure they do not exceed budgets.
Activity Level
A measure of the volume of production or operations, usually influencing costs and revenues in managerial accounting.
Q8: List the components in an oxoacid.
Q10: In comparing three solutions with pHs of
Q20: Give two important reasons why there is
Q29: Which type of radiation is the least
Q59: Complete and balance the following redox equation.
Q76: You have 500.0 mL of a buffer
Q83: When the following equation is balanced with
Q85: What is the electron configuration of a
Q87: The following is the correct order for
Q115: A possible reaction leading to removal of