What is ΔG°rxn for the following reaction? SiCl4(g) + 2Mg(s) → 2MgCl2(s) + Si(s) 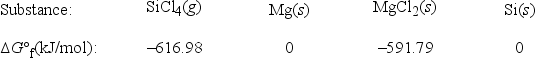
Definitions:
Suppliers Of Insurance
Entities that provide various insurance policies to individuals or organizations to protect against potential financial losses or damages.
Expected Value
A calculated average outcome of a random event, considering all possible outcomes and their probabilities.
Car Insurance
A type of insurance that provides financial protection against physical damage or bodily injury from traffic collisions and against liability that could also arise from incidents in a vehicle.
Probability
A measure of the likelihood of a particular event or outcome occurring, expressed as a number between 0 and 1.
Q1: The air at sea level is ~80%
Q28: The solubility of lead(II) iodide is 0.064
Q38: If a certain process has ΔS<sub>univ </sub>>
Q57: Ozone in the stratosphere protects animals and
Q81: Which is the half-reaction at the anode
Q83: Which statement is correct?<br>A) Heating always decreases
Q91: When a<sup>87</sup> Br nucleus emits a beta
Q113: Will a precipitate (ppt) form when 20.0
Q118: _ is the uppermost layer of the
Q127: What is the pH of a 0.056