The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide. (R = 8.314 J/K • mol) H2O2(l)  H2O2(g)
H2O2(g)
Use the following thermodynamic information at 298 K to estimate this temperature. 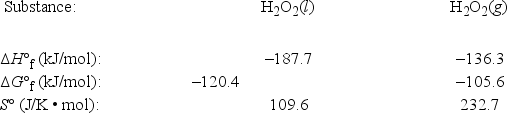
Definitions:
Backward Integration
A business strategy where a company expands its role to fulfill tasks formerly completed by businesses up the supply chain, gaining control over suppliers or production.
Horizontal Integration
A strategy used by businesses to increase their market share by acquiring or merging with competitors operating at the same level of the supply chain.
Supply-Chain Strategy
Planning and management of all activities involved in sourcing, procurement, and logistics management activities.
Vertical Integration
The expansion of a company's operations into different stages of production within the same industry, usually to increase control over the supply chain.
Q16: A(n) _ is an ion containing a
Q18: Find the pH of a 0.20 M
Q29: Write out the steps in the mechanism
Q37: What product forms at the cathode during
Q49: Carbon dioxide absorbs heat from Earth by<br>A)
Q75: The equilibrium constant for the reaction of
Q98: Explain the main reason for the temperature
Q100: A change in the temperature can change
Q113: The radioisotope potassium-40 decays to argon-40 by
Q126: What is the name of the molten