Calculate ΔG° for the combustion of ethanol vapor, C2H5OH(g) , at 750.°C in oxygen to form carbon dioxide and water vapor. The following data are valid at 25°C: 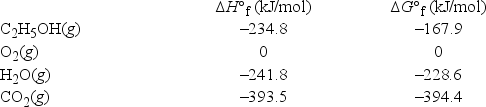
Definitions:
Chronic Illness
A long-lasting health condition that can be managed but not cured, often affecting a person's lifestyle and well-being.
Bed Bath
A method of bathing someone who is bedridden or unable to bathe themselves, typically performed with a washcloth and basin of water.
Standard Precautions
A set of infection control practices used to prevent transmission of diseases that can be acquired by contact with blood, body fluids, non-intact skin (including rashes), and mucous membranes.
Personal Protective Equipment
Personal protective equipment, or PPE, refers to protective clothing, helmets, gloves, face shields, goggles, facemasks, and/or respirators or other equipment designed to protect the wearer from injury or infection.
Q25: Calculate the minimum concentration of Cr<sup>3+</sup> that
Q34: If E° for X + e<sup>-</sup> →
Q60: What is the value of the equilibrium
Q77: _ is the major mechanism by which
Q91: If 50.0 mL of 1.2 × 10<sup>-3
Q94: What are magic numbers with reference to
Q94: A(n) _ can be added to a
Q98: Consider this reaction at equilibrium at a
Q117: What is ΔG° at 200°C for the
Q133: In which of these gas-phase equilibria is