Multiple Choice
Below are energy level diagrams for two different atoms. 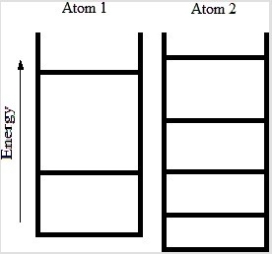 Based on the diagrams, which atom has the larger standard molar entropy at T = 298 K?
Based on the diagrams, which atom has the larger standard molar entropy at T = 298 K?
Definitions:
Related Questions
Q17: What is ΔS° at 25°C for the
Q18: Suppose 75.0 g of PCl<sub>5</sub>(g) is introduced
Q37: Which is the best choice for preparing
Q37: Ozone protects us from ultraviolet light by<br>A)
Q56: Which one of these species exists as
Q74: In the equation below, what particle or
Q93: The element oxygen was prepared by Joseph
Q97: Propanoic acid (CH<sub>3</sub>CH<sub>2</sub>COOH) has a K<sub>a</sub> of
Q101: _ is used as a subscript beside
Q105: Which response includes all of the following