What is ΔS° at 298 K for the following reaction? Fe2O3(s) + 3CO(g) → 3CO2(g) + 2Fe(s) 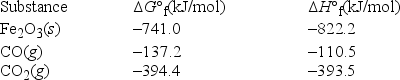
Definitions:
Net Present Value
A valuation method that calculates the present value of an investment's expected cash flows, minus the initial investment cost.
Monthly Interest Rate
The percentage of interest that is charged or earned on a loan or investment over a month's time period.
Credit Policy
A set of guidelines that a company follows to determine credit terms for customers, such as payment period and discount rates.
Discounted Price
Discounted Price refers to a price that has been reduced from the original or listed cost, often to accelerate sale or clearance or as part of promotional offers.
Q5: What is ΔS° for the reaction SO<sub>2</sub>(s)
Q8: What are the primary pollutants from automobile
Q18: If an endothermic process is spontaneous when
Q31: Which compound has the highest solubility in
Q32: Changing the amount of reactant or product
Q36: For the reaction BrO<sub>3</sub><sup>- </sup>+ 5Br<sup>- </sup>+
Q69: A galvanic cell is constructed using the
Q76: Which of the following is emitted into
Q76: Which is the correct cell notation for
Q94: What is the equilibrium constant at 25°C