What is ΔS° for the following reaction? 2Cl2(g) + SO2(g) → SOCl2(g) + Cl2O(g) 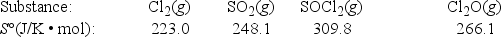
Definitions:
Push Model
A distribution method where products are pushed through the supply chain, from production to final consumers, without direct consumer demand driving it.
Pull Model
A supply chain management strategy where the demand for product initiates production and distribution, in contrast to the traditional push model driven by forecasted demand.
Open-Source CRM
A customer relationship management system whose source code is available freely for use, modification, and distribution.
On-Premise
Refers to software and technology that is installed and runs on the premises of the person or organization using the software, rather than at a remote facility.
Q36: For the reaction BrO<sub>3</sub><sup>- </sup>+ 5Br<sup>- </sup>+
Q43: Which electrochemical cell pictured below corresponds to
Q50: What is the name that refers to
Q59: Which is the strongest acid?<br>A) HBrO<sub>3</sub><br>B) HClO<br>C)
Q61: For the hypothetical reaction A + 3B
Q65: Below is a representation of an aqueous
Q81: The observation that at equilibrium, the reaction
Q86: Consider the chemical reaction 2NH<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg"
Q100: The solubility of lead(II) chloride is 0.45
Q130: The most abundant element in the Earth's