What is ΔG°rxn at 298 K for the following reaction? 2Cl2(g) + SO2(g) → SOCl2(g) + Cl2O(g) 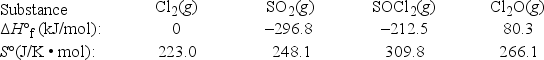
Definitions:
Quarter
One-fourth of a whole, often used to describe one of four equal parts of a year, hour, or any given quantity.
Seven Hours
A specific time duration equivalent to 420 minutes or 25,200 seconds.
Ten Hours
A period of time equating to 600 minutes or 36,000 seconds, often referenced in relation to scheduling or duration.
Aerobic Exercise
Any form of physical exercise that relies on the oxygen process, typically involving moderate intensity activities sustained over long periods.
Q4: What is the nuclear process called in
Q26: A voltaic cell consists of a Hg/Hg<sub>2</sub><sup>2+</sup>
Q48: For a process that is spontaneous at
Q57: At temperatures below 273 K, it is
Q67: The net rotation of plane-polarized light by
Q74: The reaction A + 2B → Products
Q84: Which of the following will decrease the
Q96: What is ΔS° for the following reaction?
Q107: The primary source of photochemical smog is<br>A)
Q112: The following energy-level diagram could correspond to