What is ΔG°rxn for the following reaction? SiCl4(g) + 2Mg(s) → 2MgCl2(s) + Si(s) 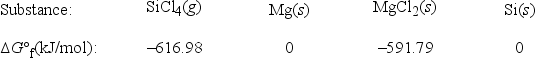
Definitions:
Physical Focus
The emphasis on or concentration of resources and efforts on bodily or material aspects of existence.
Identification
A psychological process in which an individual assimilates an aspect, property, or attribute of another and is transformed, wholly or partially, by the model the other provides.
Freud
Sigmund Freud was an Austrian neurologist who founded psychoanalysis, a method for treating psychopathology through dialogue between a patient and a psychoanalyst.
Attitudes And Values
Internalized beliefs and preferences that influence an individual's behavior and perception of the world, guiding how one evaluates issues, objects, or people.
Q17: Bromothymol blue is a common acid-base indicator.
Q40: Which is the strongest acid?<br>A) HBrO<sub>3</sub><br>B) HFO<sub>3</sub><br>C)
Q63: The equilibrium constants (expressed in atm) for
Q63: How does the entropy change when a
Q65: The _ is the solution that is
Q68: Below is a representation of the sparingly
Q90: For the following reaction at equilibrium in
Q114: What was responsible for the "orange glow"
Q117: What is the value of K<sub>b</sub> for
Q132: d and l isomers of a chiral