Calculate ΔG°rxn for the following reaction of ammonia with fluorine. 2NH3(g) + 5F2(g) → N2F4(g) + 6HF(g) 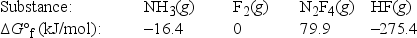
Definitions:
Achievements Layout
The organization and presentation of one's accomplishments, often in a resume or portfolio, to highlight one's skills and successes.
Combination Layout
A design approach that integrates multiple types of layouts or elements, such as text and images, for diverse or dynamic effects.
Accomplishments Layout
A structured format for presenting achievements, typically used in resumes or professional profiles.
Hard Numbers
Hard numbers are precise, quantifiable data points or statistics that are used to support claims, analysis, or decision making in a clear and objective way.
Q20: Give two important reasons why there is
Q26: Acid rain is precipitation having a pH
Q29: A buffer is prepared by adding 150
Q37: A container was charged with hydrogen, nitrogen,
Q45: Calculate the NaOH concentration necessary to precipitate
Q56: At elevated temperatures, hydrogen iodide may decompose
Q63: Which of the following does not readily
Q81: Which is the half-reaction at the anode
Q92: Acid strength decreases in the series HI
Q98: Which of the following is used to