The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide. (R = 8.314 J/K • mol) H2O2(l)  H2O2(g)
H2O2(g)
Use the following thermodynamic information at 298 K to estimate this temperature. 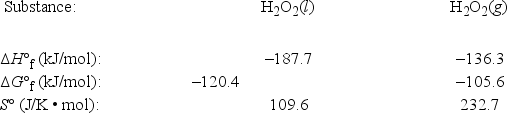
Definitions:
Hallucinations
Hallucinations are sensory perceptions that appear real but are created by the mind, without an external stimulus, affecting vision, hearing, or other senses.
Delusions
Fixed false beliefs that are resistant to reason or confrontation with actual fact, often observed in psychiatric conditions.
Coma
A state of unconsciousness in which the person shows no response to maximum painful stimuli, absence of reflexes, and absence of muscle tone in the extremities.
Traumatic Brain Injury
Damage to the brain caused by an external physical force, leading to temporary or permanent impairments in cognitive, physical, or emotional functioning.
Q10: Which one of these species is a
Q30: Which of these species is not an
Q39: Choose the incorrect statement concerning radon gas.<br>A)
Q45: What is the third law of thermodynamics?
Q47: Which of the following are common indoor
Q47: Which is the strongest acid?<br>A) HBrO<sub>4</sub><br>B) HBr<br>C)
Q66: If the system 3H<sub>2</sub>(g) + N<sub>2</sub>(g) <img
Q95: Given the following standard reduction potentials, <img
Q96: Which one of the following is not
Q111: How many minutes would be required to