Below are energy level diagrams for two different atoms. 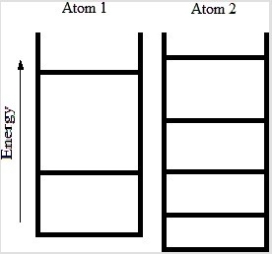 Based on the diagrams, which atom has the larger standard molar entropy at T = 298 K?
Based on the diagrams, which atom has the larger standard molar entropy at T = 298 K?
Definitions:
Variable Overhead Rate Variance
The difference between the actual variable overhead costs incurred and the standard cost of the variable overhead that should have been incurred based on the actual activity level.
Critical Part
Critical Part refers to a component or element of a system that is essential to its function, where failure could lead to a system stoppage or serious defect.
Commercial Airline Seats
The seats available for purchase on a commercial aircraft, categorized into various classes like economy, business, and first class.
Standard Labor-Hours
The expected amount of time that it should take to produce one unit of a product or complete a process.
Q26: A voltaic cell consists of a Hg/Hg<sub>2</sub><sup>2+</sup>
Q37: What product forms at the cathode during
Q41: What is the concentration of H<sup>+</sup> in
Q43: At 450°C, tert-butyl alcohol decomposes into water
Q68: How many different ways can water vibrate?
Q81: The observation that at equilibrium, the reaction
Q100: Below is a representation of pure liquid
Q100: The compound CFCl<sub>3</sub> is used as a/an<br>A)
Q103: List three primary pollutants removed from automobile
Q121: The only stable isotope of aluminum is