Suppose 50.0 mL of a 0.100 M solution of the weak acid HA is titrated with a 0.100 M solution of NaOH. Which diagram best represents the equilibrium composition of the solution at point X on the titration curve below? (Solvent water molecules are omitted for clarity.) 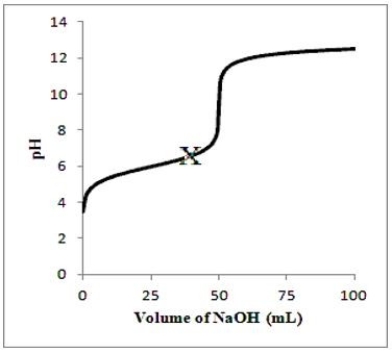
Definitions:
Sensory Interaction
The principle that one sense can influence or change how another sense is perceived, demonstrating the complex interplay between different sensory modalities.
Strawberry Odor
A distinctive sweet and fruity fragrance characteristic of strawberries, often replicated in foods and scented products.
McGurk Effect
A perceptual phenomenon demonstrating an interaction between hearing and vision in speech perception, where visual information affects what we hear.
Anosmia
A condition characterized by the loss of the ability to detect one or more smells.
Q4: The equilibrium constant, K<sub>P</sub>, has a value
Q11: The _ law of thermodynamics states that
Q32: Changing the amount of reactant or product
Q34: Consider the reaction N<sub>2</sub>(g) + 3H<sub>2</sub>(g) <img
Q55: A 35.0-mL sample of 0.20 M LiOH
Q56: Which is a basic oxide?<br>A) NO<sub>2</sub><br>B) H<sub>2</sub>O<br>C)
Q73: The entropy change ΔS° at 298 K
Q75: The equilibrium constant for the reaction of
Q99: A patient's thyroid gland is to be
Q133: For a conjugate acid-base pair, K<sub>w</sub> =