Below is a representation of an aqueous solution of a weak acid HA at equilibrium. (Each circle represents 1.0 ×10-3 mol of atoms, and the volume of the box is 1.0 L. Solvent water molecules are not shown for clarity.) 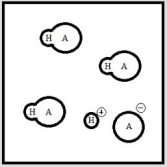 What is the pH of the solution?
What is the pH of the solution?
Definitions:
Marginal Tax Rate
The rate at which the last dollar of a taxpayer’s income is taxed, indicating the percentage of additional income that will be taxed.
Average Tax Rate
The portion of total income that is paid as taxes, calculated by dividing the total amount of taxes paid by the total income.
Tax Liability
The total amount of tax that an individual or business owes to the government, based on their income or revenue.
Marginal Tax Rate
The rate at which the last dollar of income is taxed, representing the percentage of tax applied to your income for each tax bracket in which you qualify.
Q15: A wildlife biologist is interested in testing
Q43: Ammonium chloride is used as an electrolyte
Q48: At 340. K, K<sub>P</sub> = 69 for
Q50: An "ideal solution" is a solution that
Q82: Which process defines how an ionic compound
Q83: What is the pH of a buffer
Q85: As a chemical reaction proceeds toward equilibrium,
Q93: Does a precipitate of magnesium fluoride form
Q96: Dissolving a solute such as KOH in
Q108: Sodium carbonate, Na<sub>2</sub>CO<sub>3</sub>(s), may be prepared by