Below is a representation of an aqueous solution of a weak acid HA at equilibrium. (Each circle represents 1.0 ×10-3 mol of atoms, and the volume of the box is 1.0 L. Solvent water molecules are not shown for clarity.) 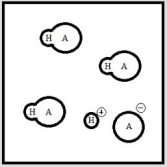 What is the pH of the solution?
What is the pH of the solution?
Definitions:
Probability Distribution
A mathematical function that describes the likelihood of obtaining the possible values that a random variable can assume.
Uncertainty
The state of having limited knowledge where it is impossible to exactly describe an existing state or future outcome.
Specific Risk
Risk affecting a narrow segment of the market, often associated with individual companies or industries, distinct from broad market risk.
Beta
In finance, a measure of a stock's volatility relative to the overall market.
Q56: A plot of the number of neutrons
Q63: What are the small molecules that constitute
Q71: The most acidic oxides are formed from
Q78: What is the name given to the
Q79: What is the pH of a 0.20
Q92: Acid strength decreases in the series HI
Q114: The endpoint is used to estimate the
Q123: A(n) _ occurs when a compound containing
Q129: Suppose 3.0 ×10<sup>-3</sup> mol of NaOH are
Q132: A solution is 40.00% by volume benzene