Multiple Choice
Below is a representation of an aqueous solution of a weak acid HA at equilibrium. (Each circle represents 1.0 mmol of atoms, and the volume of the box is 1.0 L. Solvent water molecules are not shown for clarity.) 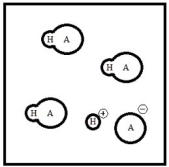 What is the percent ionization of HA?
What is the percent ionization of HA?
Definitions:
Related Questions
Q3: If a 100-mL sample of a volatile
Q19: Which of the following states that the
Q32: Reduction occurs at the anode of a
Q37: The distinguishing characteristic of all nonelectrolyte solutions
Q56: When the following redox equation is balanced
Q64: Calculate the pH of a buffer solution
Q64: The units of the rate of reaction
Q71: An alpha particle is a helium atom.
Q107: The distinguishing characteristic of all electrolyte solutions
Q115: The osmotic pressure of a 0.82 M