Hydrogen sulfide can be formed in the following reaction: H2(g) + 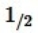 S2(g)
S2(g)  H2S(g) ; ΔH°rxn = -92 kJ/mol
H2S(g) ; ΔH°rxn = -92 kJ/mol
The equilibrium constant,KP,is 106 at 1023 K. Estimate the value of KP at 1218 K. (R = 8.314 J/K • mol)
Definitions:
Highly Integrated
Being highly integrated refers to systems or processes that are closely interconnected and work seamlessly together to achieve a common goal.
Major Oil Companies
Large, international companies that are involved in the extraction, production, refinement, and distribution of oil.
Environmental Uncertainty
The degree to which future conditions or outcomes are unpredictable within the context of an organization's external environment, influencing strategic planning and decision-making.
Resource Dependence
A theory that posits that organizations depend on resources beyond their control, which forces them to engage in behaviors to acquire and maintain these resources.
Q9: The endpoint in a titration is defined
Q23: What is meant by the prefix "poly"?<br>A)
Q31: Which compound has the highest solubility in
Q51: The isomerization of methyl isocyanide, CH<sub>3</sub>NC →
Q60: What is ΔS° for the following reaction?
Q87: Which of the following is defined as
Q106: Consider the following equilibria: 2SO<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg"
Q106: What name is given to a minor
Q113: Acid strength increases in the series HCN
Q125: Butyric acid is responsible for the odor