For the endothermic reaction A2(g)  2A(g) , a snapshot of an equilibrium mixture of A(g) and A2(g) may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.)
2A(g) , a snapshot of an equilibrium mixture of A(g) and A2(g) may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.) 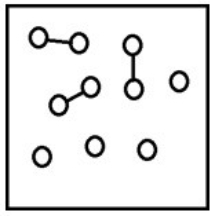 What is the equilibrium constant Kc for this reaction at 298 K? (R = 0.08206 L • atm/K • mol)
What is the equilibrium constant Kc for this reaction at 298 K? (R = 0.08206 L • atm/K • mol)
Definitions:
Sales Returns
Goods returned by customers post-purchase, leading to a reversal of sales revenue.
Net Sales
Total revenue from sales minus returns, allowances, and discounts, representing the actual sales revenue earned.
Perpetual Inventory System
An accounting system that records inventory transactions in real time, immediately affecting the inventory account with each purchase or sale.
Gross Method
An accounting method for recording purchases of inventory with no immediate recognition of discounts; discounts taken are recorded as income.
Q13: The reaction A + 2B → Products
Q15: The reaction of methane with water to
Q18: Calculate the percent by mass of potassium
Q19: Consider the figure below which shows ΔG°
Q51: What is the pOH of a 0.025
Q55: For the endothermic reaction A<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg"
Q61: The pH of a Ba(OH)<sub>2</sub> solution is
Q76: The following reaction is spontaneous under standard
Q114: For the reaction C<sub>6</sub>H<sub>14</sub>(g) → C<sub>6</sub>H<sub>6</sub>(g) +
Q115: Which is a Lewis acid?<br>A) NH<sub>3</sub><br>B) NH<sub>4</sub><sup>+</sup><br>C)