Consider the following potential energy profile for the A → B reaction. Assuming that the frequency factor for the reaction is 5.5 × 1010 s-1, what is the rate constant of this first-order reaction at 310 K? 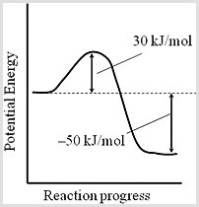
Definitions:
Q4: Which of the following compounds is appreciably
Q22: Phosgene, COCl<sub>2</sub>, a poisonous gas, decomposes as
Q28: The following diagram represents the first-order decomposition
Q40: Tetrafluoroethylene, C<sub>2</sub>F<sub>4</sub>, can be converted to octafluorocyclobutane,
Q72: What is the pH of a 0.050
Q88: What is the half-life for a zeroth-order
Q93: Describe the chemicals used to make a
Q97: Propanoic acid (CH<sub>3</sub>CH<sub>2</sub>COOH) has a K<sub>a</sub> of
Q102: Gases are sold in large cylinders for
Q124: The molar enthalpy of vaporization of hexane