The following is an Arrhenius plot of a first-order reaction. The rate constant is measured in units of s-1. 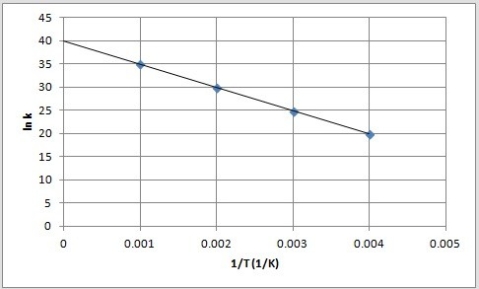 Based on this Arrhenius plot, what is the activation energy of the reaction? (R = 8.314 J/K •mol)
Based on this Arrhenius plot, what is the activation energy of the reaction? (R = 8.314 J/K •mol)
Definitions:
Budget Constraint
The limitation on the consumption bundles that a consumer can afford based on their income and the prices of goods and services.
Net Demands
The total demand for a product or service minus the supply provided, reflecting the actual market demand not met by current production.
Gross Demands
The total demand for a good or service before accounting for substitutions or complementarity effects.
Budget Line
A budget line represents all the combinations of two goods that a consumer can purchase with a given budget, given the prices of the goods.
Q16: Which one of the following crystallizes in
Q26: A 50.0-mL sample of a 0.100 M
Q29: _ is the process used to stabilize
Q36: Which compound has the lowest solubility in
Q49: If 25.5 L of oxygen are cooled
Q64: The units of the rate of reaction
Q81: The observation that at equilibrium, the reaction
Q86: What is the molarity of a solution
Q86: Which of the following is a polymer?<br>A)
Q118: Based on the phase diagram of a