The following is an Arrhenius plot of a first-order reaction. The rate constant is measured in units of s-1. 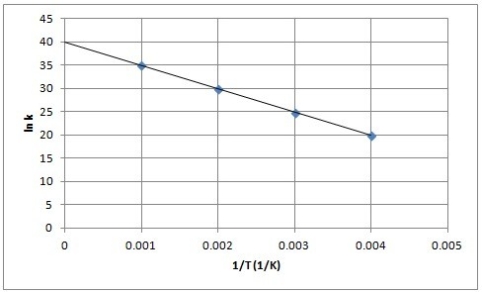 Based on this Arrhenius plot, what is the Arrhenius frequency factor (A) of the reaction? (R = 8.314 J/K mol)
Based on this Arrhenius plot, what is the Arrhenius frequency factor (A) of the reaction? (R = 8.314 J/K mol)
Definitions:
Confidentiality
The principle of keeping certain information private, not disclosing it without proper authorization or necessity.
Immunizations
Administration of a vaccine to protect susceptible individuals from communicable diseases.
Half Brother
A male sibling who shares one biological parent with another sibling, making them genetically half-related.
Step Brother
A male sibling gained through the marriage of one’s parent to the parent of the other child, not related by blood.
Q4: The equilibrium constant, K<sub>P</sub>, has a value
Q49: In the following diagrams, the black circles
Q53: For the overall chemical reaction shown below,
Q58: What is the conjugate acid of the
Q58: The intermediate in a reaction appears in
Q66: What is the molarity of a 17.0%
Q66: Calculate the pH of a 0.021 M
Q72: A 50.00-mL solution of 0.10 M HNO<sub>2</sub>
Q95: The most basic oxides are formed from
Q121: What is the freezing point of a