Consider the following potential energy profile for the A → B reaction. Assuming that the frequency factor for the reaction is 5.5 × 1010 s-1, what is the rate constant of this first-order reaction at 310 K? 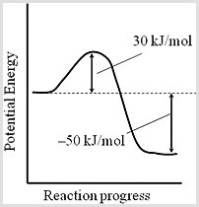
Definitions:
Top-Down Processing
A cognitive process where perception begins with the brain applying what it knows and expects to perceive and then works downward to the specifics of the input received.
Neocortical Processing
Relates to the functions of the neocortex, a part of the brain involved in higher-order brain functions such as sensory perception, cognition, and motor control.
Temporal Processing
The brain's ability to perceive and organize stimuli or events in time, including the capacity to sequence, compare, and predict temporal relationships.
Cranial Nerves
Twelve pairs of nerves that emerge directly from the brain, not the spinal cord, and supply the head and neck with motor and sensory innervation.
Q23: Nitrosyl chloride (NOCl) decomposes at elevated temperatures
Q26: A 50.0-mL sample of a 0.100 M
Q30: What is the integrated rate law for
Q39: Which one of the following is a
Q66: A sample of carbon dioxide gas at
Q68: When a reaction system reaches equilibrium, the
Q69: Consider the weak bases below and their
Q69: When the concentrations of both the reactants
Q85: If 2.38 mol of a gas has
Q101: For the chemical reaction A → C,