The following is an Arrhenius plot of a first-order reaction. The rate constant is measured in units of s-1. 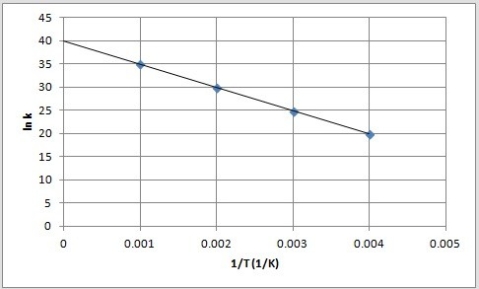 Based on this Arrhenius plot, what is the activation energy of the reaction? (R = 8.314 J/K •mol)
Based on this Arrhenius plot, what is the activation energy of the reaction? (R = 8.314 J/K •mol)
Definitions:
Marketing Segmentation
The process of dividing a market into distinct subsets of consumers with similar needs or characteristics, allowing for targeted marketing strategies.
Different Groups
Distinct categories or clusters in which data points are divided based on specific characteristics or treatments.
Confidence
The feeling of trust or belief in someone or something; in statistics, it refers to the degree to which one can be certain in the accuracy of a result.
Proportion
A part, share, or number considered in comparative relation to a whole; a type of ratio.
Q5: For the following reaction at equilibrium in
Q22: Phosgene, COCl<sub>2</sub>, a poisonous gas, decomposes as
Q28: The following diagram represents the first-order decomposition
Q31: A sample of an ideal gas has
Q42: Which is more soluble in an acidic
Q42: The isomerization of cyclopropane follows first-order kinetics.
Q65: The activation energy for the following first-order
Q71: The number of atoms in a face-centered
Q109: "The pressure of an ideal gas is
Q111: The air pressure in a volleyball is