The following is an Arrhenius plot of a first-order reaction. The rate constant is measured in units of s-1. 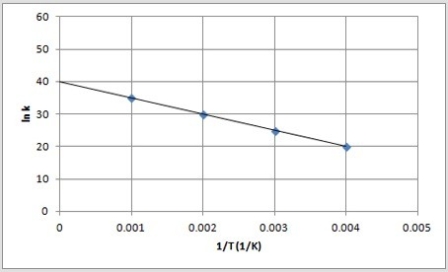 If a catalyst is added to the reaction, which could correspond to an Arrhenius plot of the catalyzed reaction?
If a catalyst is added to the reaction, which could correspond to an Arrhenius plot of the catalyzed reaction?
Definitions:
Logistical Function
Tasks associated with procuring, manufacturing, and disseminating goods and products in correct volumes to a target user or end point.
Assorting
The process of selecting and grouping complementary products or items to offer for sale, aimed at maximizing sales and customer convenience.
Transporting
The movement of goods or individuals from one location to another through various modes of transportation such as trucks, trains, ships, or airplanes.
Rogerian Psychotherapy
A client-centered approach to therapy developed by Carl Rogers, emphasizing unconditional positive regard, empathy, and genuineness in the therapeutic relationship.
Q3: Weak acids have weak conjugate bases.
Q11: Which of the following aqueous solutions should
Q45: A sample of solid naphthalene is introduced
Q52: Iodine trichloride, ICl<sub>3</sub>, will react with a
Q55: For the endothermic reaction A<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg"
Q58: For which of the following pure substances
Q79: What is defined as the difference between
Q86: What is the molarity of a solution
Q133: A solution is prepared by adding 0.10
Q136: For the following reaction at equilibrium, which