Below is a diagram representing a solvent A(l) in a 1-L beaker, and a solute X dissolved in the solvent. Solvent A has a density of 0.8 g/mL, and a molar mass of 40 g/mol. Solute X has a molar mass of 30 g/mol. Each circle of X represents 1 mol of X. Assume that the solute addition does not significantly change the volume of liquid in the beaker. 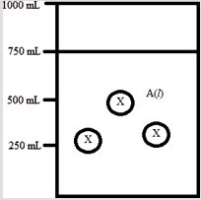 What is the mass percent of solute X in this solution?
What is the mass percent of solute X in this solution?
Definitions:
Ethics
The moral principles that govern a person's behavior or the conducting of an activity, often guiding decision-making and actions in a professional setting.
Code of Ethics
A set of principles and rules designed to guide behavior and decision-making within an organization or profession.
Organizational Values
The core principles and beliefs that guide the actions and behaviors of an organization and its members, defining its culture and ethical stance.
Ethical Principles
Fundamental guidelines or moral values that dictate the right and wrong ways of conducting oneself or performing actions.
Q3: Weak acids have weak conjugate bases.
Q7: What is another name given to dispersion
Q19: The rate law for the rearrangement of
Q20: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 × 10<sup>-3</sup>,
Q38: At 400ºC, K<sub>c</sub> = 64 for the
Q43: Which response lists all the following pairs
Q44: Which substance should exhibit hydrogen bonding in
Q54: The _ is the equation relating the
Q81: What is the formula which describes the
Q123: Consider the general reaction 5Br<sup>-</sup>(aq) + BrO<sub>3</sub><sup>-</sup>(aq)