Consider the following reaction. H2(g) + I2(s) → 2 HI(g)
If the cylinder with a movable piston below contains 1 mol H2(g) and 1 mol I2(s) , which represents the cylinder after the reaction is complete? 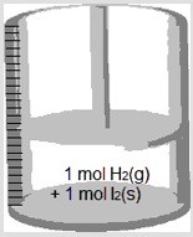
Definitions:
Paroxysmal Nocturnal Dyspnea
A condition characterized by sudden, severe shortness of breath at night, often waking the person from sleep, commonly associated with heart failure.
Tachycardia
A condition where the heart rate exceeds the norm for an individual's age, resting at over 100 beats per minute.
Orthopnea
Difficulty in breathing that occurs when lying down and is typically relieved by sitting or standing up, often associated with heart failure.
Hypoxia
A condition in which the body or a part of the body is deprived of adequate oxygen supply at the tissue level.
Q26: A gas cylinder containing 1.50 mol compressed
Q29: _ is the process used to stabilize
Q56: _-_ in rubber makes the rubber much
Q60: _ is the formula to determine the
Q65: A flask containing neon gas is connected
Q71: Which molecule has the largest dipole moment?<br>A)
Q73: What types of elements undergo covalent bonding?<br>A)
Q73: The electron configuration of a ground-state vanadium
Q97: Which is true concerning the molecular geometry
Q106: Atomic orbitals developed using quantum mechanics<br>A) describe