What is ΔH°rxn for the following reaction? SiO2(s) + 4HCl(g) → SiCl4(g) + 2H2O(g) 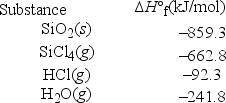
Definitions:
Specialty Line
A range of products or services that cater to a particular niche market, usually distinguished by their high quality or uniqueness.
Market
A place or mechanism where buyers and sellers meet to engage in exchange of goods and services.
Atmospherics
The physical elements of a store or business environment designed to influence a consumer's mood and behavior.
Emotional Manipulation
The use of psychological tactics to influence others' feelings and behaviors for one's own advantage, often undermining the individual's choices or well-being.
Q39: If normal body temperature is 98.6°F then
Q42: If an element is bonded to 4
Q49: What volume of a 0.442 M NaOH
Q49: Determine the number of protons, electrons, and
Q101: Using Hess' law, what is ΔH°<sub>rxn </sub>at
Q123: What is the electron configuration for tungsten?<br>A)
Q127: What is the mass percent of oxygen
Q128: Select the compound in which sulfur has
Q131: Tetraphosphorus hexaoxide is formed by the reaction
Q136: Given that CaO(s) + H<sub>2</sub>O(l) → Ca(OH)<sub>2</sub>(s),