An important step in the synthesis of nitric acid is the conversion of ammonia to nitric oxide according to the following balanced chemical equation. What is ΔH°rxn for this reaction? 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g) 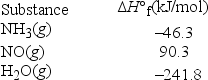
Definitions:
Wage Rates
The standard amount of pay given to workers per unit of time (hourly, daily, etc.) for their labor.
Labor Inputs
The work effort provided by employees that is used in the production process of goods and services.
AFC
Average Fixed Cost, which is the fixed cost of production divided by the quantity of output produced, illustrating how fixed costs dilute over larger production volumes.
AVC
AVC stands for Average Variable Costs, referring to the total variable costs of production divided by the quantity of output produced, indicating how variable costs per unit change with output levels.
Q7: Which field of study made a big
Q31: How many electrons are there in the
Q38: The elements in Group 7A are known
Q49: Each of the noble gases has a
Q63: Calculate the molecular mass of tetraphosphorus decaoxide,
Q74: Which molecule has a Lewis structure that
Q75: What is the name of P<sub>4</sub>Se<sub>3</sub>?<br>A) phosphorus
Q83: How many dots does the Lewis dot
Q92: For which reaction is ΔH approximately (or
Q110: Pentaborane B<sub>5</sub>H<sub>9</sub>(s) burns vigorously in O<sub>2</sub> to