The bond enthalpy of the Br-Cl bond is equal to ΔH°rxn for the following reaction. BrCl(g) → Br(g) + Cl(g)
Using the following data, what is the bond enthalpy of the Br-Cl bond? 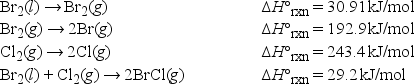
Definitions:
Network Effects
Increases in the value of a product to each user, including existing users, as the total number of users rises.
Price Discrimination
The strategy of selling the same product to different customers at different prices based on willingness to pay, rather than differences in production costs.
"Fair Return" Price
A price set at a level intended to allow a firm to cover its costs and earn a reasonable profit, often regulated in public utility sectors.
Q6: If aqueous solutions of Ba(OH)<sub>2</sub> and HNO<sub>3</sub>
Q16: The _ is the one component that
Q24: What is the empirical formula for a
Q57: The general electron configuration for atoms of
Q60: Which type of system may transfer energy,
Q74: Which member of the halogen family has
Q80: Vinegar is a solution of acetic acid,
Q102: The heat absorbed by a system at
Q122: Ammonia reacts with diatomic oxygen to form
Q137: Phenolphthalein is a universal indicator and maybe