Suppose a large piece of a metallic solid, A(s) , is placed in a beaker containing the metallic cation Z2+(aq) , as depicted below. 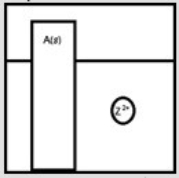 If Z is a more active metal than A, which is a possible representation of the final reaction mixture?
If Z is a more active metal than A, which is a possible representation of the final reaction mixture? 
Definitions:
Retailers
Businesses that sell goods or commodities directly to consumers, often from physical locations or through online platforms.
Credit Policies
Credit policies refer to the guidelines and standards set by a business regarding the extension of credit to customers, including terms, conditions, and criteria for creditworthiness.
Monthly Rate of Return
The percentage gain or loss on an investment over a one-month period.
Variable Cost
Expenses that change in proportion to the activity of a business.
Q10: List some recent microbiology discoveries of great
Q20: A geology student found an irregularly shaped
Q47: Who is credited with discovering the atomic
Q60: A 50.0 mL sample of 0.436 M
Q81: What is ΔH°<sub>rxn</sub> for the decomposition of
Q94: What is the oxidizing agent in the
Q101: Calculate the number of moles in 17.8
Q102: The percent yield can be determined by
Q110: Based on the solubility rules, which of
Q126: A 6.0-gram champagne cork is shot out