The figure below represents what type of differentiation? 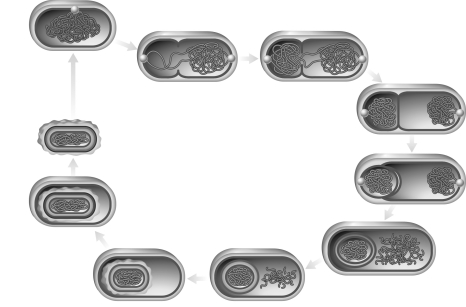
Definitions:
Atomic Number
The number of protons in the nucleus of an atom, which determines the chemical properties of an element and its place in the periodic table.
Mass Number
The total number of protons and neutrons in the nucleus of an atom, representing the atom's mass.
Electronegativity
A chemical property that describes the tendency of an atom to attract a shared pair of electrons towards itself in a covalent bond.
Polarity
A property of having distinct and opposite ends or sides, often seen in molecules with uneven distribution of charges.
Q9: Metabolism of a microbe is best examined
Q10: Retroelements are remains of retroviral genomes in
Q20: The most common form of chemical energy
Q23: With optimistic locking,locks are first issued,then the
Q25: Atomic force microscopy measures _ between a
Q34: Which of the following forms of transposition
Q41: Which of the following is NOT true
Q64: The highest useful magnification for a light
Q71: Which of the following is NOT an
Q86: Hadoop is a(n):<br>A)RDMBS.<br>B)OODBMS.<br>C)distributed file system (DFS).<br>D)print system.<br>E)Web