Consider the following molecules. What is the relationship between the degree to which the molecule is oxidized and its polarity? 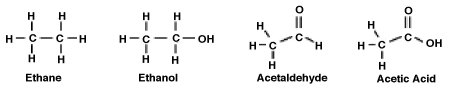
Definitions:
Non-electrolytes
Substances that do not produce ions when dissolved in water, and hence do not conduct electricity in solution.
Non-electrolyte
A substance that does not readily ionize when dissolved or melted and is therefore a poor conductor of electricity.
Dissociate
In chemistry, it refers to the process by which molecules, ions, or complexes split into smaller particles or ions, often reversible and influenced by environmental conditions.
Aqueous Solution
A mixture where water acts as the dissolving medium.
Q2: In humans, the maintenance of a warm,
Q12: What is one role of unpaired valance
Q17: Which of the following describes the path
Q32: How do protein hormones and steroid hormones
Q36: If DNA is described as resembling a
Q46: Which of the following reactions illustrates an
Q51: Nanotechnology looks at working with particles the
Q70: Atoms of nonmetallic elements form covalent bonds,
Q80: What is the main difference between a
Q209: If you had a 1 M solution