Consider the following molecules. What is the relationship between the degree to which the molecule is oxidized and its polarity? 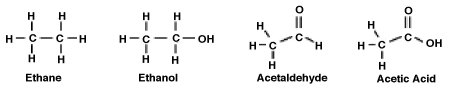
Definitions:
Interstate Commerce
Economic activities or transactions that cross state borders or affect more than one state, which are regulated by the federal government in the United States.
Single-Family Farm
A farm operated and maintained by a single family, usually for the purposes of sustenance or income.
RPM Agreement
A Resale Price Maintenance agreement, where manufacturers or wholesalers set the minimum prices at which retailers can sell their products.
Per Se Rule
Legal doctrine stating that an action or condition is inherently illegal or violates a law in itself, without the need for additional proof.
Q16: Neurons that connect neurons to other neurons
Q19: Why might coating a metal with another
Q25: Which of the above molecules is a
Q37: Why are ion-dipole attractions stronger than dipole-dipole
Q53: If an alcoholic beverage is 20 percent
Q53: Which would you expect to have a
Q74: Which of the following would not be
Q79: Microtechnology began<br>A)about 60 years ago with the
Q80: For the following reaction, identify whether the
Q103: Which should be larger, the potassium atom,