Consider the boiling points of the following compounds and their solubilities in room-temperature water. Why does the solubilities in water go down as the boiling points of these alcohols go up. 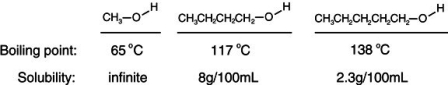
Definitions:
Analytical Intelligence
One of three facets of Robert J. Sternberg's triarchic theory of intelligence, focusing on the ability to analyze, evaluate, judge, compare, and contrast.
Metacognition
An awareness and understanding of one’s own thought processes, often referred to as "thinking about thinking."
Problem Solving
The process of identifying solutions to difficult or complex issues through analytical thought, creativity, and logical reasoning.
Distributive Justice
The principle concerned with the fair allocation of resources among diverse members of a community.
Q1: Complete the following nuclear equation: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8398/.jpg"
Q2: Which of the above illustrations shows a
Q3: Why is the combination of two protons
Q9: Which of the following describes a nonmetal?<br>A)poor
Q47: Which of the following molecules would contain
Q76: Radium-226 is a common isotope on Earth,
Q92: Carbon-carbon single bonds can rotate while carbon-carbon
Q110: What property primarily determines the effect of
Q154: Which of the following is a pure
Q219: How many covalent bonds would the following