Consider the boiling points of the following compounds and their solubilities in room-temperature water. Why does the solubilities in water go down as the boiling points of these alcohols go up. 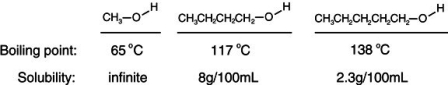
Definitions:
Motive State Excitement
The arousal or stimulation of a particular motivational state, leading to increased activity or response.
Need for Stimulation
is a psychological concept suggesting that individuals seek experiences and situations that offer sensory or cognitive stimulation.
Exploratory Drive
is the innate drive or motivation behind exploration and discovery, prompting individuals to seek new information, experiences, or environments.
Curiosity
A strong desire to know or learn something, driving exploration and investigation.
Q25: How many different elements are in the
Q27: If the following generic atom were to
Q30: What is the main tenet of Plank's
Q70: A floating leaf oscillates up and down
Q73: Formaldehyde is a toxic preservative with the
Q86: Which of the following statements best describes
Q101: For the following reaction, identify whether the
Q184: How do the electron-dot structures of elements
Q197: Which of the following molecules has the
Q249: When a hydronium ion concentration equals 1