The boiling point of 1,4-butanediol is 230°C. Would you expect this compound to be soluble or insoluble in room-temperature water? 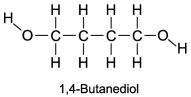
Definitions:
Perfectly Price Discriminate
A situation where a seller charges each buyer their maximum willingness to pay, capturing all consumer surplus as profit.
Surplus
A condition where the supply of a product or service surpasses its demand at the existing price.
Monopolist
A Monopolist is a market participant who has exclusive control over the market for a particular good or service, facing no competition.
Perfectly Price Discriminate
A theoretical pricing strategy where a seller charges the maximum possible price that each consumer is willing to pay.
Q28: How do fission power plants work?<br>A)The heat
Q32: Which of the following material phases cannot
Q41: Rust has a tendency to form when
Q108: Which of the following elements is a
Q115: If an element has 18 protons and
Q117: Is the mass of an atomic nucleus
Q118: If you were to increase the pH
Q137: Magnesium ions carry a 2+ charge, and
Q181: If the solubility of a compound is
Q195: The charges with sodium chloride are all