Use the graph to determine the following limits, and discuss the continuity of the function at x = -4. (i) 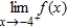 (ii)
(ii) 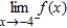 (iii)
(iii) 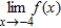
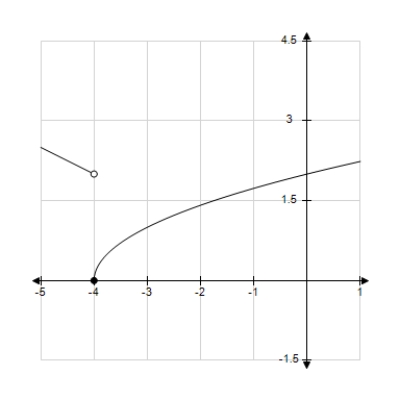
Definitions:
Secondary Carbons
A carbon atom bound to two other carbon atoms within an organic molecule, often influencing the molecule's reactivity and physical properties.
Trimethylheptane
A saturated hydrocarbon with the formula C10H22; it’s a branched alkane used in organic synthesis and as a reference in octane rating.
Carbon Atoms
The basic building blocks of the vast majority of organic compounds, capable of forming strong covalent bonds due to their tetravalency.
Hydrogen Atoms
The simplest type of atoms consisting of one proton and one electron, fundamental in chemistry and the building blocks of molecules.
Q14: Find the limit. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8527/.jpg" alt="Find
Q36: Find the vertical asymptotes (if any) of
Q59: Find the minimum distance from the point
Q70: Find the area of the portion of
Q75: Find three positive numbers x, y, and
Q93: Find the derivative of the function <img
Q97: Let <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8527/.jpg" alt="Let be
Q131: Find an equation to the tangent line
Q139: Which of the following functions passes through
Q160: Find <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8527/.jpg" alt="Find at